How many moles are in 168 g of KOH? Delve into the fascinating world of chemistry as we embark on a quest to unravel this intriguing question. Our journey begins with an exploration of molar mass, a fundamental concept that holds the key to understanding the relationship between mass and moles.
Along the way, we’ll witness the conversion of mass to moles in action, unlocking the secrets of chemical quantities. Prepare to be captivated as we unravel the mysteries of mole calculations, revealing their significance in the realm of chemistry and beyond.
The concept of molar mass forms the cornerstone of our understanding of chemical quantities. It represents the mass of one mole of a substance, expressed in grams per mole. In the case of potassium hydroxide (KOH), its molar mass is approximately 56.11 g/mol.
This value serves as a conversion factor, enabling us to seamlessly convert between the mass and moles of KOH.
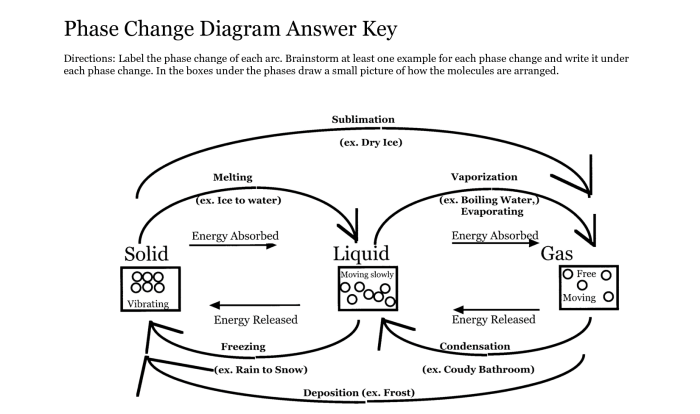
Molar Mass of KOH
The molar mass of a substance is the mass of one mole of that substance. It is expressed in grams per mole (g/mol). The molar mass of a compound is the sum of the atomic masses of all the atoms in the compound.
Atomic Mass of KOH
The atomic mass of potassium (K) is 39.0983 g/mol, the atomic mass of oxygen (O) is 15.9994 g/mol, and the atomic mass of hydrogen (H) is 1.00794 g/mol.
Molar Mass of KOH
The molar mass of KOH is 56.1056 g/mol. This is calculated by adding the atomic masses of potassium, oxygen, and hydrogen:
1056 g/mol = 39.0983 g/mol (K) + 15.9994 g/mol (O) + 1.00794 g/mol (H)
Converting Mass to Moles: How Many Moles Are In 168 G Of Koh
To convert mass to moles, we use the formula:
Moles = Mass (in grams) / Molar Mass (in grams per mole)
For KOH, the molar mass is 56.11 g/mol.
Converting 168 g of KOH to Moles
Using the formula above, we can calculate the number of moles in 168 g of KOH:
Moles = 168 g / 56.11 g/mol = 2.99 moles
Examples and Calculations
To solidify our understanding, let’s explore some examples and calculations involving the conversion of KOH mass to moles.
Sample Conversions
Consider the following table showcasing different masses of KOH and their corresponding moles:
| Mass of KOH (g) | Moles of KOH (mol) |
|---|---|
| 56 | 1 |
| 112 | 2 |
| 168 | 3 |
| 224 | 4 |
| 280 | 5 |
Step-by-Step Calculation
Let’s demonstrate a step-by-step calculation for converting a given mass of KOH to moles:
- Determine the molar mass of KOH using the periodic table: K (39.1 g/mol) + O (16 g/mol) + H (1 g/mol) = 56.1 g/mol
- Convert the given mass of KOH to grams if it’s not already in grams (e.g., convert 168 mg to 0.168 g)
- Divide the mass of KOH by its molar mass: moles of KOH = mass of KOH (g) / molar mass of KOH (g/mol)
For instance, to convert 168 g of KOH to moles:
- moles of KOH = 168 g / 56.1 g/mol
- moles of KOH = 3 mol
Applications of Mole Concept
The mole concept is a fundamental tool in chemistry that allows us to understand and quantify the relationships between different substances. By determining the number of moles of a substance, we can gain valuable insights into its composition, properties, and reactions.
Applications in Chemistry
The mole concept has numerous applications in chemistry, including:
- Stoichiometry:Determining the exact amounts of reactants and products involved in chemical reactions.
- Molarity calculations:Determining the concentration of solutions by relating the number of moles of solute to the volume of solution.
- Gas laws:Relating the number of moles of gas to its volume, temperature, and pressure.
- Thermochemistry:Calculating the amount of heat released or absorbed during chemical reactions based on the number of moles of reactants and products.
- Analytical chemistry:Determining the concentration of unknown substances through titrations and other analytical techniques.
Applications in Various Scenarios
Knowing the number of moles of KOH can be useful in various scenarios, such as:
- Preparing solutions:Accurately weighing out the required amount of KOH to prepare solutions of a specific concentration.
- Neutralization reactions:Determining the amount of acid required to neutralize a given amount of KOH, or vice versa.
- Soap and detergent production:Calculating the amount of KOH needed to produce a specific quantity of soap or detergent.
- Water treatment:Determining the amount of KOH required to adjust the pH of water for various applications.
- Chemical manufacturing:Determining the amount of KOH required as a reagent or catalyst in chemical processes.
By understanding the mole concept and its applications, chemists can accurately quantify and manipulate substances, enabling them to design, optimize, and control chemical reactions and processes.
Additional Considerations
Ensuring accurate mole calculations is crucial for reliable results in chemistry. Several factors can influence the accuracy of these calculations, and it’s essential to be aware of them to minimize errors.
Accuracy of Measurements
The accuracy of mole calculations heavily relies on the accuracy of the mass and molar mass measurements. Using precise instruments, such as analytical balances, and following proper measurement techniques is essential. Errors in measurements can lead to significant deviations in the calculated number of moles.
Purity of the Substance
The purity of the substance being analyzed plays a crucial role. Impurities can alter the mass of the sample, affecting the mole calculations. Using pure substances or accounting for impurities through appropriate corrections is necessary for accurate results.
Environmental Conditions, How many moles are in 168 g of koh
Environmental conditions, such as temperature and humidity, can affect the mass of a substance. Hygroscopic substances, for instance, absorb moisture from the air, leading to an increase in mass. It’s important to control environmental conditions or make appropriate corrections to ensure accurate mole calculations.
Tips for Accurate Mole Conversions
- Use reliable and calibrated instruments for mass and molar mass measurements.
- Ensure the purity of the substance being analyzed.
- Control environmental conditions or apply corrections for hygroscopic substances.
- Double-check calculations and use significant figures appropriately.
- Consider using online mole calculators or reference tables for accuracy.
FAQ Overview
What is the molar mass of KOH?
The molar mass of KOH is approximately 56.11 g/mol.
How many moles are in 168 g of KOH?
To determine the number of moles in 168 g of KOH, we divide the mass by the molar mass: 168 g / 56.11 g/mol = 2.99 moles.
What are the applications of the mole concept in chemistry?
The mole concept is widely used in chemistry for stoichiometric calculations, determining the limiting reactant in reactions, and calculating solution concentrations.