Draw the structure of 1 3 dichloropropane – Draw the structure of 1,3-dichloropropane, a versatile organic compound with diverse applications. This guide delves into its structural representation, IUPAC nomenclature, isomerism, physical and chemical properties, synthesis, reactions, and practical uses.
Structural Representation
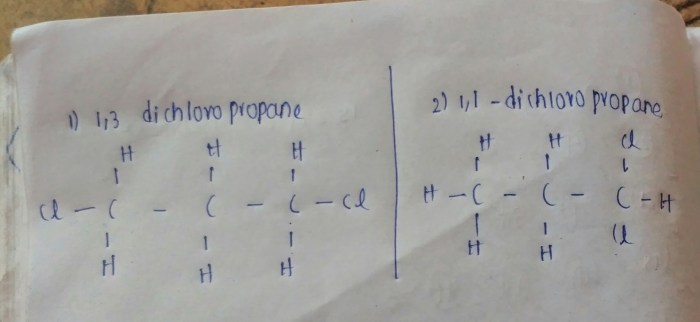
1,3-dichloropropane can be represented structurally as:
CH3-CHCl-CH 2Cl
Each carbon atom is labeled with the number of hydrogen atoms attached to it.
IUPAC Nomenclature
The IUPAC name for 1,3-dichloropropane is 1,3-dichloropropane.
The root word “prop” indicates a three-carbon chain. The prefixes “di” and “chloro” indicate the presence of two chlorine atoms. The suffix “-ane” indicates that the compound is an alkane.
Other compounds with similar IUPAC names include:
- 1,2-dichloroethane
- 1,4-dichlorobutane
- 2,3-dichloropentane
Isomerism

1,3-dichloropropane has two isomers:
- 1,3-dichloropropane
- 1,2-dichloropropane
The two isomers differ in the position of the chlorine atoms on the carbon chain.
1,3-dichloropropane is the more stable isomer because the chlorine atoms are farther apart. This reduces the steric hindrance between the chlorine atoms.
Physical and Chemical Properties: Draw The Structure Of 1 3 Dichloropropane
1,3-dichloropropane is a colorless liquid with a boiling point of 117 °C and a melting point of -97 °C. It is denser than water and insoluble in water.
1,3-dichloropropane is a reactive compound. It can undergo a variety of reactions, including substitution, addition, and elimination reactions.
Synthesis and Reactions
1,3-dichloropropane can be synthesized by the reaction of propene with chlorine gas.
CH3-CH=CH 2+ Cl 2→ CH 3-CHCl-CH 2Cl
1,3-dichloropropane can also be synthesized by the reaction of 1,2-dichloropropane with sodium hydroxide.
CH3-CHCl-CH 2Cl + NaOH → CH 3-CHCl-CH 2OH + NaCl
1,3-dichloropropane can undergo a variety of reactions, including:
- Substitution reactions
- Addition reactions
- Elimination reactions
Applications
1,3-dichloropropane is used as a solvent, degreaser, and intermediate in chemical synthesis.
It is a good solvent for oils, greases, and waxes. It is also used as a degreaser for metal surfaces.
1,3-dichloropropane is an intermediate in the synthesis of a variety of other compounds, including:
- 1,3-propanediol
- 1,3-dichloropropene
- 1,3-dichloropropanol
FAQ Summary
What is the IUPAC name for 1,3-dichloropropane?
1,3-Dichloropropane
How many isomers does 1,3-dichloropropane have?
Two
What is the boiling point of 1,3-dichloropropane?
96.8 °C