Amoeba sisters properties of water answer key – Unveiling the intricate properties of water, this guide explores the fascinating realm of Amoeba Sisters’ comprehensive exploration. Dive into the polarity of water molecules, the significance of hydrogen bonding, and the remarkable specific heat capacity that shapes its behavior. Embark on a journey through the three states of water, unraveling the processes of evaporation, condensation, and freezing.
Discover the remarkable ability of water as a solvent, dissolving various substances with remarkable ease. Delve into the vital role of water in biological systems, sustaining life and driving cellular processes. Finally, explore the environmental significance of water, its distribution, and the impact of human activities on this precious resource.
Properties of Water
Water, the elixir of life, possesses unique properties that make it essential for sustaining life on Earth. Understanding these properties is crucial for comprehending its vital role in various scientific disciplines.
Polarity of Water Molecules
Water molecules are polar, meaning they have a slightly positive end (hydrogen atoms) and a slightly negative end (oxygen atom). This polarity arises from the uneven distribution of electrons, creating a dipole moment.
Hydrogen Bonding in Water
Hydrogen bonding is a strong intermolecular force that occurs between water molecules due to their polarity. The positive end of one molecule attracts the negative end of another, forming a network of hydrogen bonds. This network contributes to water’s unique properties.
High Specific Heat Capacity
Water has a high specific heat capacity, meaning it requires a significant amount of energy to raise its temperature. This property enables water to act as a temperature buffer, stabilizing the temperature of its surroundings.
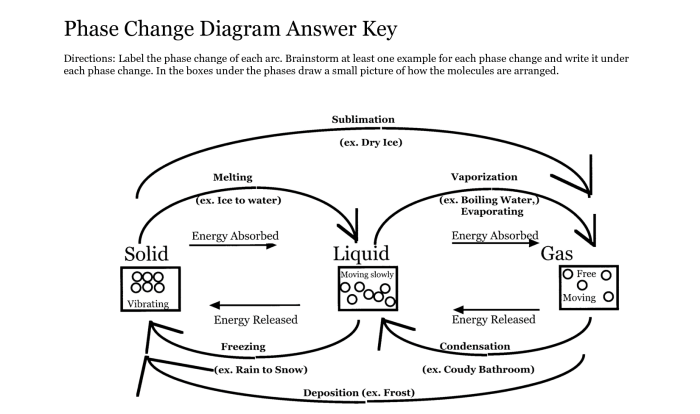
States of Water: Amoeba Sisters Properties Of Water Answer Key
Water exists in three states under standard conditions: solid (ice), liquid (water), and gas (water vapor). These states are determined by temperature and pressure.
Evaporation, Condensation, and Freezing
Evaporation is the process by which liquid water transforms into water vapor, while condensation is the reverse process. Freezing occurs when liquid water cools and solidifies into ice.
Factors Affecting Boiling and Freezing Points
The boiling point of water is the temperature at which it turns into vapor, while the freezing point is the temperature at which it turns into ice. These points are influenced by factors such as atmospheric pressure and the presence of impurities.
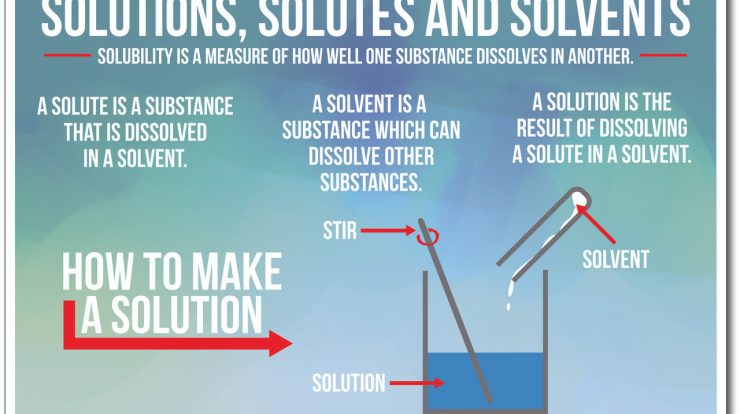
Water as a Solvent
Water is an excellent solvent due to its polarity. It can dissolve a wide range of substances, both ionic and covalent. The polarity of water molecules allows them to interact with charged particles and polar molecules, forming solvation spheres.
Ionic and Covalent Compounds
Ionic compounds, such as sodium chloride, dissolve in water by dissociating into their ions. Covalent compounds, such as sugar, dissolve in water by forming hydrogen bonds with water molecules.
Water in Biological Systems

Water is essential for life on Earth, constituting approximately 60-70% of the human body. It plays a vital role in various cellular processes, including metabolism, transport, and waste removal.
Cellular Processes
Water is involved in numerous cellular processes, such as enzymatic reactions, solute transport, and maintaining cell shape.
Water Potential
Water potential is a measure of water’s tendency to move from one area to another. It is influenced by factors such as solute concentration and pressure, and plays a crucial role in plant water relations.
Environmental Significance of Water
Water is a precious resource that is unevenly distributed across the globe. Human activities have a significant impact on water quality and quantity, posing challenges to water security.
Distribution of Water on Earth, Amoeba sisters properties of water answer key
Water covers approximately 71% of Earth’s surface, but only a small fraction is freshwater available for human use.
Impact of Human Activities
Pollution, over-extraction, and climate change are major threats to water resources, affecting both water quality and availability.
Water Conservation and Management
Water conservation and management are essential for ensuring sustainable water use and preserving this vital resource for future generations.
Helpful Answers
What is the significance of hydrogen bonding in water?
Hydrogen bonding is responsible for water’s unique properties, including its high surface tension, high specific heat capacity, and ability to dissolve many substances.
How does water’s polarity affect its solvent properties?
Water’s polarity allows it to dissolve both ionic and covalent compounds. Ionic compounds dissociate into ions when dissolved in water, while covalent compounds form hydrogen bonds with water molecules.
Why is water essential for life?
Water is essential for life because it is involved in many important cellular processes, including metabolism, transport, and temperature regulation.