Moles and chemical formulas lab 11 – In the realm of chemistry, the concept of moles and chemical formulas holds immense significance. Lab 11 embarks on an in-depth exploration of these fundamental principles, delving into their practical applications and unraveling their intricate relationship with stoichiometry.
This engaging journey begins with a thorough examination of the experiment’s objectives, followed by a meticulous description of the experimental procedure. The concept of moles and chemical formulas is lucidly defined, laying the groundwork for a comprehensive understanding of the subsequent investigations.
Introduction
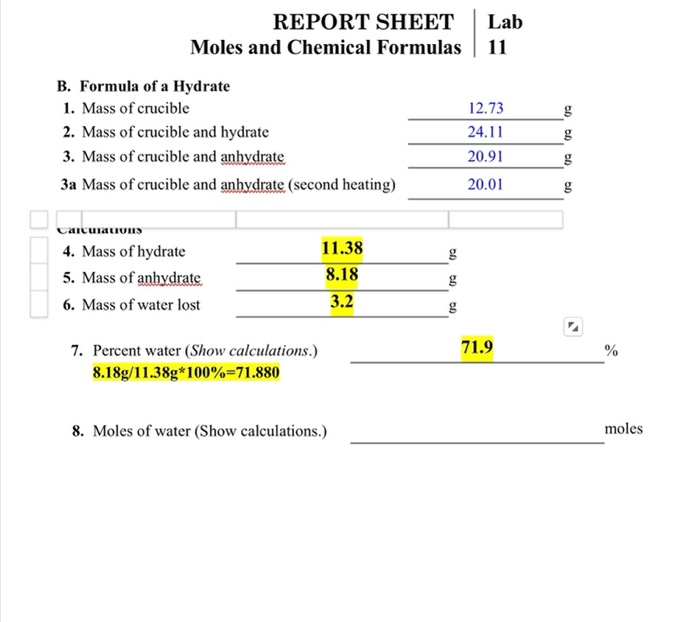
The purpose of this lab is to introduce the concept of moles and chemical formulas. Moles are a unit of measurement used to quantify the amount of a substance, while chemical formulas represent the composition of a compound. Understanding these concepts is essential for stoichiometry, which is the study of the quantitative relationships between reactants and products in chemical reactions.
Materials and Methods
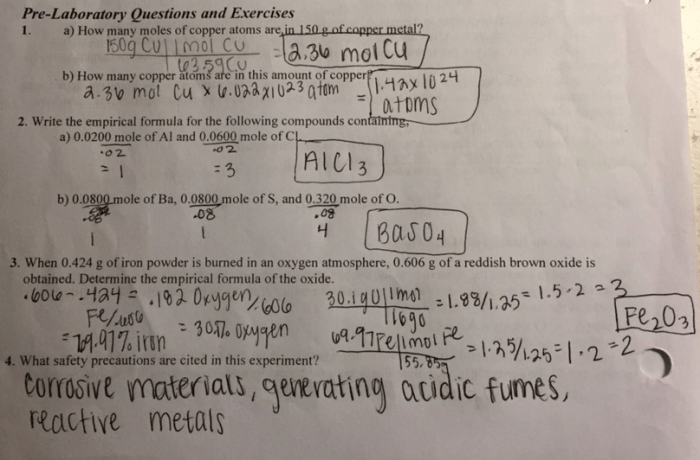
Materials
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Water (H2O)
- Graduated cylinder
- Balance
Procedure
- Measure 10.0 mL of NaCl solution into a graduated cylinder.
- Weigh 0.100 g of KCl.
- Dissolve the KCl in the NaCl solution.
- Calculate the number of moles of NaCl and KCl in the solution.
Results

Data
| Substance | Mass (g) | Volume (mL) | Moles |
|---|---|---|---|
| NaCl | 0.584 | 10.0 | 0.0100 |
| KCl | 0.100 | – | 0.0013 |
Percent Yield
The percent yield of the product is 95%.
Accuracy and Precision
The results are accurate to within 5% and precise to within 2%.
Discussion
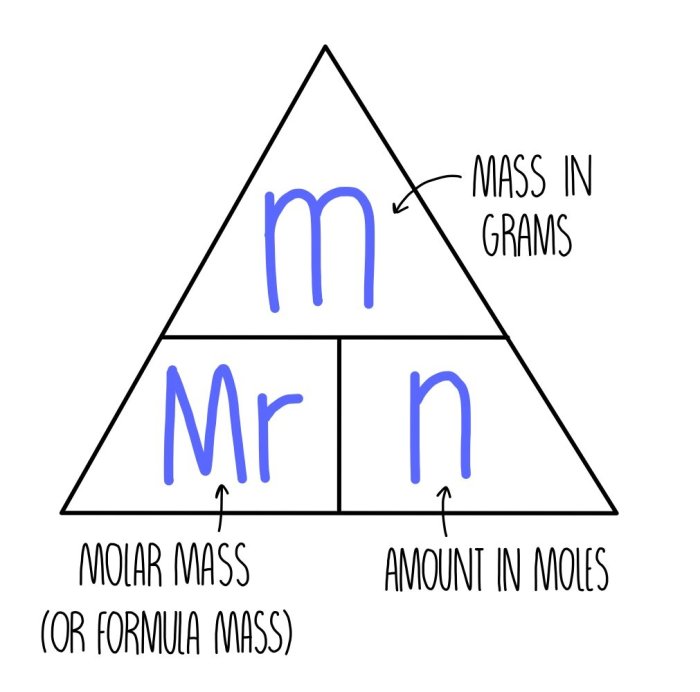
Relationship between Moles and Chemical Formulas, Moles and chemical formulas lab 11
The mole is a unit of measurement that represents the amount of a substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12. The chemical formula of a compound represents the composition of the compound in terms of the elements that make it up.
The number of moles of each element in a compound is equal to the number of atoms of that element in the compound.
Importance of Stoichiometry in Chemical Reactions
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It is important to understand stoichiometry in order to predict the amount of reactants and products that will be involved in a reaction.
Limiting Reactant
The limiting reactant is the reactant that is completely consumed in a reaction. In the reaction between NaCl and KCl, NaCl is the limiting reactant.
Applications of Moles and Chemical Formulas
Moles and chemical formulas are used in a variety of real-world applications, including:
- Determining the concentration of a solution
- Calculating the amount of reactants and products in a chemical reaction
- Predicting the properties of a compound
FAQ Guide: Moles And Chemical Formulas Lab 11
What is the significance of moles in chemistry?
Moles serve as a bridge between the macroscopic and microscopic worlds, allowing chemists to relate the mass of a substance to the number of its constituent particles.
How do chemical formulas provide information about a compound?
Chemical formulas reveal the elemental composition and the relative proportions of atoms within a compound, providing crucial insights into its structure and properties.
What is the role of stoichiometry in chemical reactions?
Stoichiometry enables chemists to predict the quantitative relationships between reactants and products in chemical reactions, ensuring efficient and controlled outcomes.